
Gas hydrates (or clathrate hydrates) are ice-like crystalline molecular complexes formed from mixtures of water and suitably sized ‘guest’ gas molecules. The water (host) molecules, upon hydrogen bonding, form lattice structures with several interstitial cavities. The guest gas molecules can occupy the lattice cavities, and when a minimum number of cavities are filled, the crystalline structure will become stable and solid gas hydrates will form, even at temperatures well above the melting point of water ice. When gas hydrates dissociate (melt), the crystalline lattice breaks down into liquid water (or converts to ice if conditions are below the freezing point of water) and the gas is released. The classic image of gas hydrates is the ‘burning snowball’ – methane hydrate supporting its’ own combustion.
As shown in the figure below, the two common forms of gas hydrates are known as structures-I and II, which have been investigated with X-ray diffraction methods by von Stackelberg and Müller (1954). They found that the unit cell of structure-I (sI) is a 12 Å cube consisting of 46 water molecules which has two types of cavities. The two small cavities are pentagonal dodecahedra (512), whereas the six large cavities are tetradecanhedra (51262) having two opposite hexagonal faces and twelve pentagonal faces. The smaller cavities are almost spherical, whereas the larger cavities of structure-I are slightly oblate. The unit cell of structure-II (sII), which is a 17.3 Å cube with 136 water molecules, also contains two types of cavities. The 16 smaller cavities are distorted pentagonal dodecahedra and the 8 larger cavities are hexadecahedra (51264) having 4 hexagonal faces and twelve pentagonal faces. The latter cavities are almost spherical in shape.
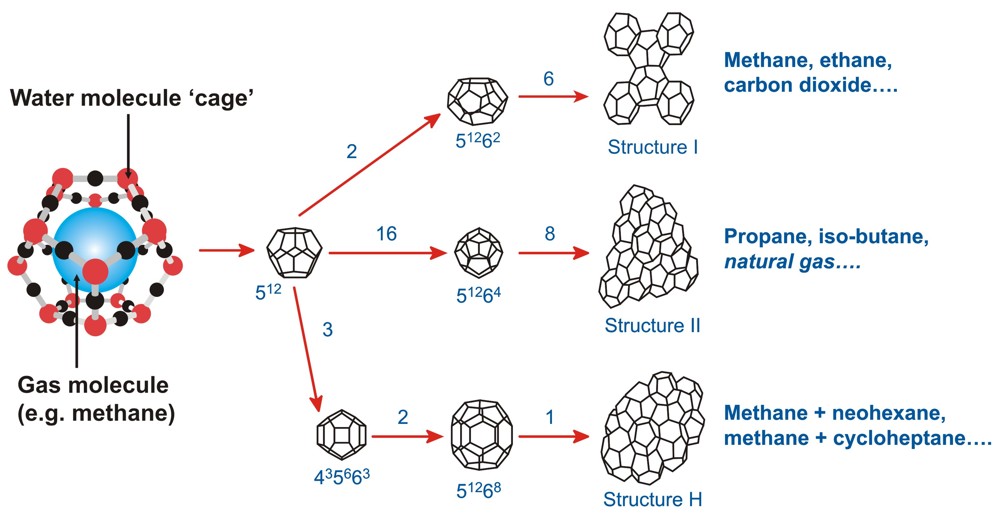
Ripmeester et al. (1987) reported a new hexagonal hydrate structure which requires both large and small molecules to stabilise the structure. They proposed that the new structure be known as structure-H (sH). According to the above authors, the unit cell has 34 water molecules forming a hexagonal lattice with parameters a = 12.26 Å and c = 10.17 Å. The structure-H has three different types of cavities, three 512 cavities which is common to all known hydrate structures, two new 12 faces 435663 cavities and one new large 51268 cavity. The 435663 cavity has three square faces, six pentagonal faces, and three hexagonal faces, whereas the 51268 cavity has 12 pentagonal faces and eight hexagonal faces. The first two cavities accommodate the small molecules which act as help gases. The large cavity in this structure can accommodate even larger molecules, so molecules in the size range of 7.5 to 8.6 Å can potentially form gas hydrates.
References
Ripmeester, J. A., Tse, J. S, Ratcliffe, C. I., and Powell, B. M. (1987): A New Clathrate Hydrate Structure, Nature, 325, 135. von Stackelberg, M. and Müller, H. R. (1954): Feste Gashydrate, Z. für Elektrochemie, 58, 25.
Suggested Reading
There are many Journal and Conference publications covering all aspects of gas hydrates available in the literature. See our publications page for details. We recommend the following books for a good summary of the main issues:
Henriet, J.-P. and Mienert, J. (eds.), Gas Hydrates: Relevance to World Margin Stability and Climatic Change, Geological Society of London Special Publication 137 (1998). Max, D., Natural Gas Hydrate in Oceanic and Permafrost Environments, Kluwer Academic Publishers, Dordrecht, Netherlands (2000). Sloan, E.D., Clathrate Hydrates of Natural Gases, Marcel Dekker Inc., New York (1998).
